GUANGZHOU, China, March 4, 2024 /PRNewswire/ — March 4th 2024 marks the 7th International HPV Awareness Day, a global campaign proposed by the International Papillomavirus Society (IPVS) since 2018. This year, the theme is One Less Worry. On this special day, Hybribio (300639.SZ) calls for raising international HPV awareness and adopting effective strategies for early detection and prevention.
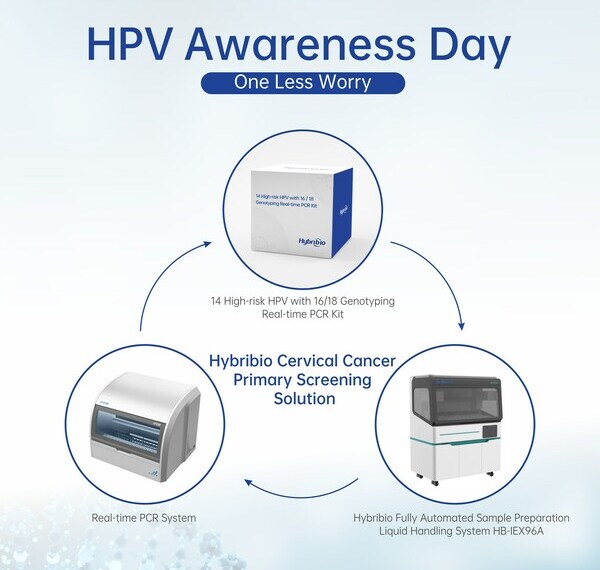
 Hybribio Cervical Cancer HPV Primary Screening Solution
Hybribio Cervical Cancer HPV Primary Screening Solution
According to the World Health Organization (WHO), there is an estimated amount of 625,600 women and 69,400 men suffering from HPV-related cancers each year. Persistent HPV infection with high-risk HPV types is the predominant cause of cervical cancer and also has a close association with other types of cancers including oropharyngeal, anal, vulvar, vagina, and penile cancers. In this case, raising HPV awareness, getting timely vaccination and high-quality screening together contribute significantly to the prevention of HPV infection and related diseases.
In 2020, the World Health Assembly adopted a global strategy to accelerate the elimination of cervical cancer. Additionally, on the path towards cervical cancer elimination, WHO raised a 90-70-90 target that must be met by all countries by 2030, which states that 70% of women should receive at least one HPV molecular test by the age of 35, and another one by 45. As the pioneer and advocator of HPV DNA testing in China, Hybribio has been promoting HPV DNA testing as the primary screening for cervical cancer and participating in women’s cervical cancer screening programs both in urban and rural areas since 2007. From 2009-2011, Hybribio organized over 600 training sessions for clinicians domestically to raise public awareness of HPV infection, screening and treatment. As of March 2024, Hybribio has provided over 67 million HPV DNA tests for females, ultimately safeguarding countless lives.
Hybribio has developed a complete HPV molecular diagnostic solution covering 14 High-risk HPV GenoArray Diagnostic Kit, 14 High-risk HPV with 16/18 Genotyping Real-time PCR Kit, 14 High-risk HPV E6/E7 mRNA Real-time PCR Kit (PCR with Fluorescent Probe), 21 HPV GenoArray Diagnostic Kit, 37 HPV GenoArray Diagnostic Kit, 13 High-risk HPV Real-time PCR Kit, 23 HPV Genotyping Real-time PCR Kit, SOX1 and PAX1 Methylation Real-time PCR Kit. In June 2023, Hybribio 14 High-risk HPV with 16/18 Genotyping Real-time PCR kit obtained the firstly approved NMPA cervical cancer primary screening license, marking a milestone of high-performance and high-standard HPV DNA testing for cervical cancer primary screening in China. With Hybribio Fully Automated Sample Preparation Liquid Handling System HB-IEX96A and commercial general Real-time PCR system, it can achieve highly efficient large-scale cervical cancer primary screening.
In addition to HPV diagnostic reagents, Hybribio has been working on HPV-related medication. The Hybribio chloroquine gel (in development) has been awarded multiple invention patents in countries (e.g. U.S.A., Russia, Japan), which is intended for the treatment of low-risk HPV infections, including skin warts such as metatarsal warts, flat warts, genital warts and perianal condyloma acuminatum, etc.
Raising international HPV awareness is the very first step against cervical cancer. It requires the participation of different parties from hospitals, enterprises, organizations, and government authorities to all women individuals. Hybribio endeavors to provide cutting-edge HPV diagnostic solutions and is committed to eliminating cervical cancer. Get vaccination, get screening, get treatment. Together we can triumph HPV!
About Hybribio
Founded in 2003, Hybribio (300639.SZ) is a leading nucleic acid test supplier and manufacturer who specializes in in-vitro diagnostic products with a fully integrated one-step operation chain from R&D, manufacturing, and sales & marketing to after-sales technical support services. Hybribio provides products including, but not limited to, women’s health, reproductive health, genetic diseases, methylation, respiratory tract infection, pharmacogenetics and instrumentation.
For more information, please visit: www.hybribio.com
This content was prepared by our news partner, Cision PR Newswire. The opinions and the content published on this page are the author’s own and do not necessarily reflect the views of Siam News Network